FDA expected to approve mixing-and-matching COVID-19 vaccines for booster shots
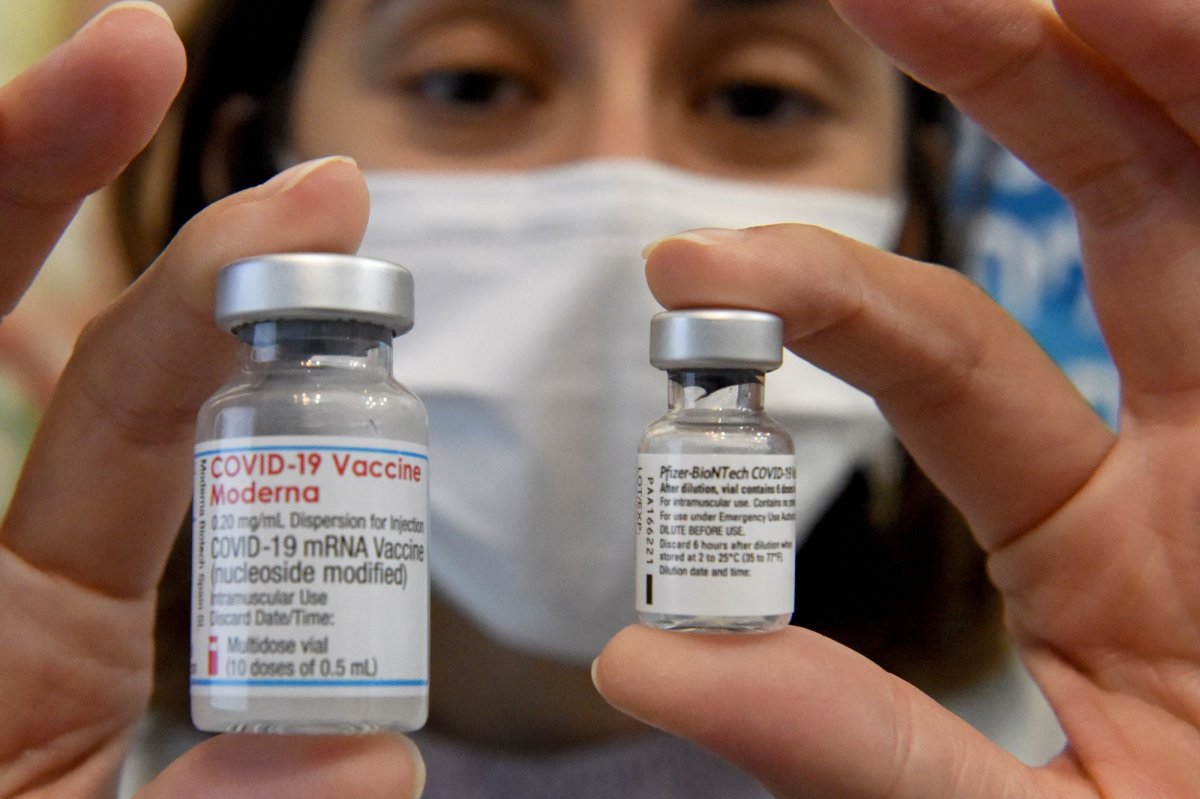
The Food and Drug Administration is expected to approve mixing-and-matching COVID-19 vaccine booster shots later this week.
The FDA already approved third doses of the Pfizer vaccine and is expected to approve the Moderna and Johnson & Johnson vaccines for booster shots shortly, as well. For example, if all three were approved for boosters, and mixing-and-matching was approved, a person who received two doses of the Moderna vaccine last spring could receive a booster from any of the three vaccines.
The Centers for Disease Control and Prevention’s advisers are scheduled to meet Thursday to discuss Moderna and J&J boosters.
Mixing-and-matching would allow greater flexibility and lead to shorter wait times for appointments to receive a booster. However, the new guidance is expected to indicate that it’s preferable to receive a booster from the same vaccine, especially for the Pfizer and Moderna vaccines that have proved most effective against the coronavirus.

However, data has shown that booster shots dramatically increase the level of antibodies regardless of which brand a person received initially, but people who received the Johnson & Johnson vaccine and then a booster from one of the other two had the greatest increase in antibodies.
Boosters also showed good protection against the delta variant of COVID.

